
Introduction:
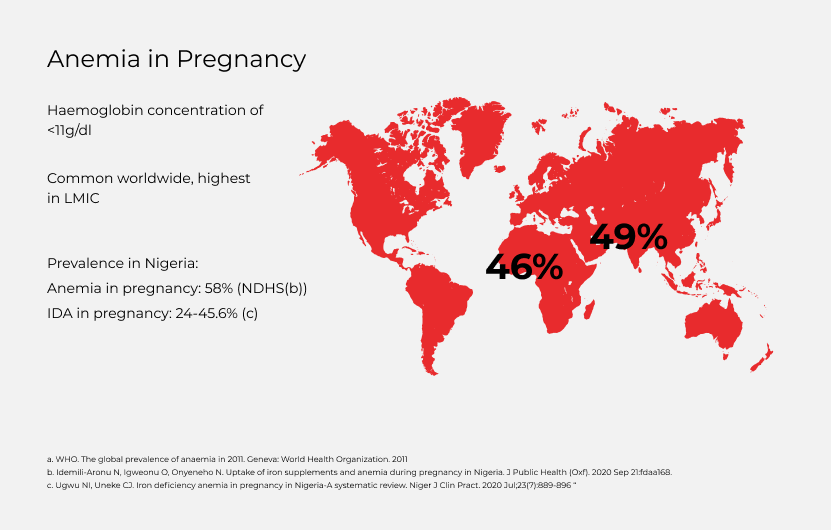
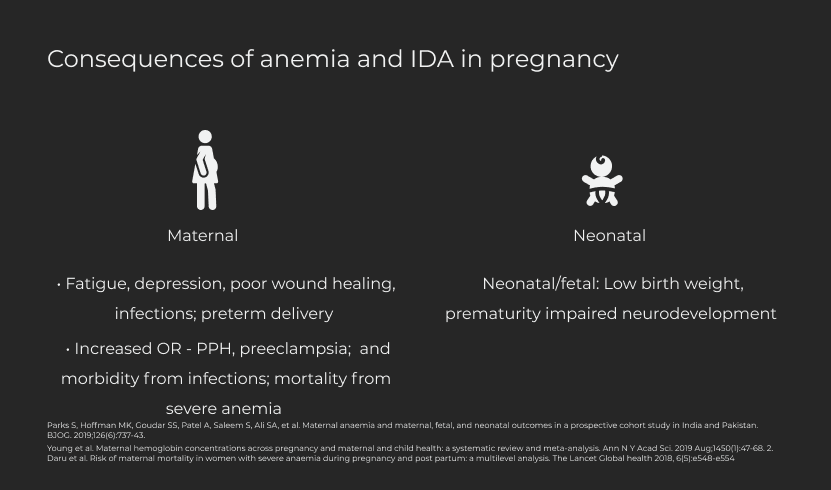
- Anaemia in pregnancy (AIP) remains a critical global health problem especially in low- and middle-income countries (LMICs), affecting 46% of pregnant women in Africa and 49% in Asia (1). AIP is defined as a venous blood haemoglobin concentration of <11 g/dl in a pregnant woman (1). Iron deficiency anaemia (IDA) is the commonest cause of AIP, accounting for 50-75% of AIP (2). Antenatal maternal anaemia has significant implications on both maternal and neonatal outcomes, including maternal mortality from haemorrhage and morbidity from infections, and neonatal low birth weight and prematurity (3). It has also been associated with an increased risk of depression (4, 5).
- Global recommendations for management of anaemia in pregnancy in LMICs advise that women be treated with high-dose daily oral iron (120 mg of elemental iron) supplementation (6). However, such high doses are often poorly tolerated due to significant gastrointestinal adverse effects such as vomiting, constipation, diarrhoea and abdominal pain, limiting adherence to this vital intervention (2, 7). In addition, delivery of oral iron during pregnancy requires ongoing contact between the pregnant woman and the healthcare facility and in settings with poor health-seeking behaviour and economic limitations, few women receive the recommended course of iron (and folic acid), leading to an increased risk of IDA in pregnancy.
- In high-income countries (HICs) and increasingly in LMICs, intravenous iron products administered in a single, rapid infusion have been used for rapid correction of anaemia (e.g. in late pregnancy, or prior to impending major surgery), and are increasingly being used for first line treatment of IDA (7, 8).
- The bioavailability and shorter-term administration and reported higher effectiveness of these intravenous products make them highly attractive for high-burden LMIC use, given the challenges of using oral elemental iron (7). However, for LMICs, it will be prudent to assess formulation safety, and to determine the efficacy and impact on mother-infant pair outcomes, especially maternal anaemia/iron status, premature delivery and infant birth weight.
- Implementation outcomes (acceptability, feasibility, fidelity), and cost effectiveness are also important.


Aims & Objectives
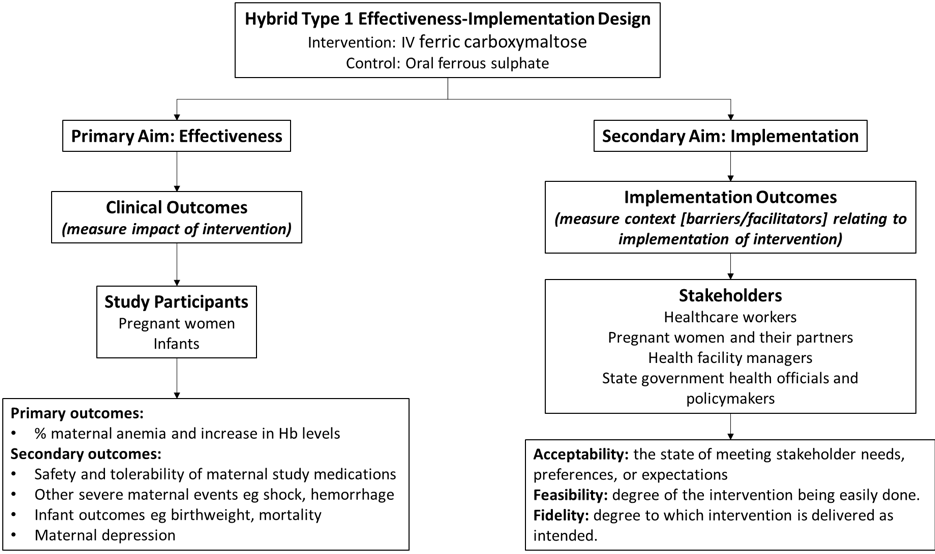
Our aim is to compare the effectiveness of intravenous ferric carboxymaltose (intervention) versus oral ferrous sulphate (control) for treating anaemia and IDA in pregnancy and to measure acceptability, feasibility, fidelity (implementation outcomes), and the cost-effectiveness of intravenous versus oral iron among pregnant Nigerian women with moderate and severe anaemia and IDA at 20–32 weeks’ gestation.
Study Outcomes
- Outcome 1: Clinical
Primary clinical outcome (Prevalence of anaemia)
The comparative effectiveness of treatment with intravenous ferric carboxymaltose (intervention) versus oral ferrous sulphate (control) in reducing the prevalence of maternal anaemia at 36 weeks’ gestation and increase in maternal haemoglobin levels at 4 weeks post-initiation of treatment and at delivery among pregnant women with iron deficiency anemia.
Secondary clinical outcomes
- The safety and tolerability of intravenous ferric carboxymaltose versus oral ferrous sulphate, including the incidence of hypophosphatemia and severity of maternal adverse effects.
- Severe maternal events, specifically, haemorrhage, sepsis, shock and the need for blood transfusion.
- The incidence of low infant birthweight (<2.5 kg), prematurity (<37 weeks’ gestation as dated from the last menstrual period or a first trimester ultrasound scan), stillbirth and neonatal mortality (birth till 28 days of life), and proportion of infants being breastfed at 1, 2 and 4 weeks of life, and receiving BCG, oral polio and hepatitis vaccination in same time period.
- Depression linked to emotional well-being of mothers using the validated Edinburgh Postnatal Depression Scale.
Outcome 2: Implementation Outcomes
To measure implementation outcomes considered most relevant and amenable to assessment in the study timeline. The measurement of these implementation outcomes is twofold: first, to guide refinement/adjustment of the study design and intervention for the prospective study (formative evaluation), and second, to gather information throughout the study timeline for documentation of any changes in study context. This will be divided into three parts:
A) Acceptability
B) Feasibility
C) Fidelity
INTRAVENOUS VERSUS ORAL IRON FOR IRON DEFICIENCY ANEMIA IN PREGNANT NIGERIAN WOMEN
Methodology
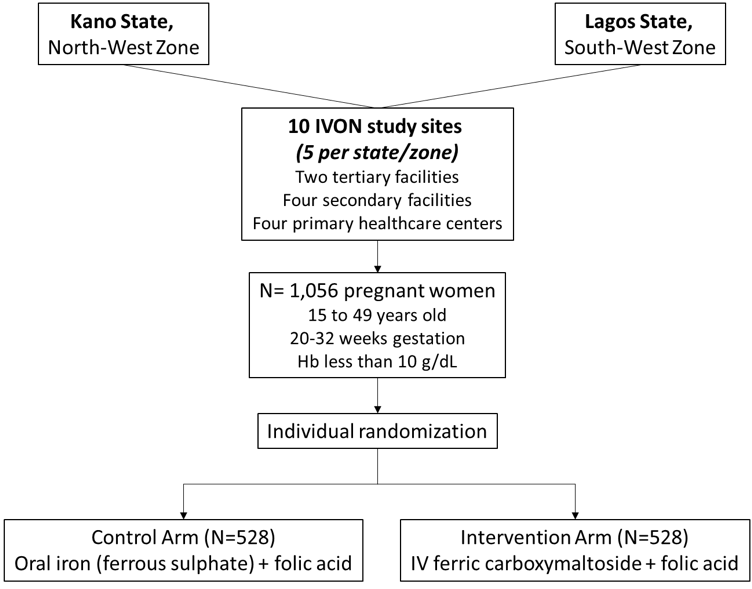
- Multicenter, parallel, open label individually randomized controlled trial, with women allocated in a 1:1 ratio in conjunction with a cost-effectiveness analysis.
- The study will be implemented in the two most populated states in Nigeria; Kano State (estimated 2016 population 13.1 million (16) in the North-West and Lagos State (estimated 2020 population 14.4 million (17) in the South-West zone.
- One tertiary, two secondary and two primary healthcare facilities will be selected from each state, making 10 targeted facilities in total.
Study Population
Pregnant women aged 15 to 49 years old between 20- and 32-weeks’ gestational age.
Intravenous versus oral iron for iron deficiency anemia in pregnant Nigerian women
Ethical Considerations
- Ethical approval obtained from the National Health Research and Ethics Committee of Nigeria, Health Research and Ethics Committees of the Lagos University Teaching Hospital, and Aminu Kano Teaching Hospital, Kano State, and the State Health Service Commissions of Lagos and Kano States.
- The trial is duly registered at the Pan African Clinical Trials Registry (PACTR), International Clinical Trials Registry (ICTR) and Nigeria Clinical Trials Registry.

IVON Trial Investigators & Sites
This is a multi-site, randomised, controlled, blind trial taking place in Lagos and Kano State
Principal Investigators & Co-Investigators
- Professor Bosede Afolabi (Principal Investigator)
- Professor Hadiza Galadanci (Co-Investigator)
- Dr. Mobolanle Balogun (Co-Investigator)
- Dr Titilope Adeyemo (Co-Investigator)
- Dr. Nadia Sam-Agudu (Co-Investigator)
- Dr Ochuwa Babah (Co-Investigator)
Study Sites
The 5 study sites in Lagos State are:
- Lagos University Teaching Hospital (LUTH), Idi-Araba
- Lagos Island Maternity Hospital, Lagos
- Mother and Child Centre, Amuwo-Odofin
- Simpson Primary Health Centre, Ebute-Metta
- Iwaya Primary Health Centre, Yaba, Lagos.
The 5 study sites in Kano state are:
- Aminu Kano Teaching Hospital, Kano
- Sheikh Jeddah General Hospital, Kano
- Bammali General Hospital, Kano
- Kumbotsu Comprehensive Primary Health Centre, Kumbotsu, Kano
- Sharada Primary Health Centre, Kano
